Go to
Johnston and Goldsmith et al. in their papers use a number of methods to selectively support the conclusions which they eventually reach. From my point of view, the authors do not disinterestedly look at the available evidence, do not make a balanced analysis, and do not present results in a neutral manner. Rather, it appears to me that the authors from the beginning support or favour a particular conclusion, and in a number of ways organise their scientific work so as to selectively support this conclusion.
This process I will call, for convenience, 'pushing of the argument'. It might also be called 'not being perfectly objective', or 'presenting one's case to best advantage', or 'being biased in favour of one's conclusion', or any one of a number of similar expressions, depending on what connotations one wished to convey.
I do not consider that demonstration of pushing in the work of a scientist should reflect unfavourably upon the competence or integrity of the scientist. I believe that pushing of scientific arguments is inevitable. Furthermore, there is no reason why pushed arguments are either unjustifiable or necessarily incorrect. The important thing is not to eliminate pushing, which is impossible anyway, but to recognise that it exists. I will present reasons in later chapters to support these beliefs. For now, let me say that I believe that the type of analysis made here of the papers of Johnston and of Goldsmith et al. could be applied to the work of all scientists to a similar if not greater degree. In other words, I believe that the work of Johnston and of Goldsmith et al. is typical of all scientific research in the degree to which the scientific arguments are pushed.
Neither does the existence of pushing automatically imply that a scientist's results or conclusions are unjustifiable or wrong. While a scientist's argument may be judged on the basis of current understanding to be pushed, it may eventually be vindicated. Or it may not. A part of scientific intuition is knowing the conclusion towards which to push a scientific argument.
Given that the pushing of arguments by scientists is not unusual, it may be asked why I have chosen the particular articles by Johnston and by Goldsmith et al. to illustrate the point. There are several reasons. First, the papers reach important, and conflicting, scientific conclusions.*
* For those who worry about priority in scientific discoveries, it is appropriate to note that many of the results and conclusions in Johnston's paper were obtained independently and perhaps slightly earlier by Crutzen, and that many aspects of Goldsmith et al.'s paper were the subject of earlier studies by Foley and Ruderman and by Johnston, Whitten and Birks. That I concentrate on the papers by Johnston and by Goldsmith et al. reflects their usefulness for my purposes, and not necessarily their importance in other contexts.
Second, these conclusions are important to all of society. For example, they have direct relevance to questions of technical development (the SST) and to interference with the environment (pollution of the stratosphere). Third, the papers, to a greater extent than the general run of scientific papers, present a full and comprehensive argument. In more specialised work many more of the assumptions made are implicit, and hence pushing of the argument is harder to recognise. I consider in chapter 8 the usefulness of Johnston's and of Goldsmith et al.'s work in this regard. Fourth, the papers appeared in Science and Nature, scientific journals which are prestigious. Therefore the papers are likely to be representative of high quality scientific research. Fifth, the journals are not highly specialised: the papers are written for the general reader with a solid scientific background. And finally, and not least importantly, the papers concern material which I have studied for several years.
Several friends suggested that I should include an analysis of other papers, in particular of papers concerning the energy analysis of nuclear reactors or reactor programmes. I agree that such a study would be fascinating, and more timely than my own. There are two main reasons why it is out of the question for me to undertake such an analysis, besides my lack of incentive to do it. First, I have had no personal research experience in the field. The study required would take years, and then the topic might be just as out of date as upper atmospheric pollution by supersonic transports is as I write this in 1976 (which is not all that out of date, but probably will be in 10 years time). Second, I did not choose the two papers primarily because of their relevance to a topical social issue. My analysis might be considered to be a facet of social and intellectual history. It just as well could have been done for papers on stellar nucleosynthesis, on methods of calculating n-body forces, or on the establishment of uniformitarian theory. The advantage of papers in an area closely related to social concerns is that pushing, and the values motivating and directing the work, are much closer to the surface, and thus easier to expose.
In the following analysis, the pushing of the arguments in the papers is considered under various categories, such as "technical assumptions" and "selective use of results". These areas are not intended to form a complete or highly accurate categorisation of the different ways in which a scientific argument may be pushed. The areas are merely used to conveniently separate the analysis into more easily comprehensible parts.
Needless to say, my analysis of the two papers itself involves pushing of the argument. In his comments on a draft of part II, Johnston thought that many of the cases where I claimed he was pushing his argument were due to other factors. Some of these factors are discussed in chapter 5. I think my arguments have merit. But it is important for you the reader to be wary of what I have to say.
Any scientific argument depends vitally upon the particular technical assumptions on which it is based. In the papers by Johnston and by Goldsmith et al. these assumptions are such things as which chemical reactions to consider, what ozone data is considered reliable and useable and how to take into account meteorological influences. Choosing one particular technical assumption rather than another can serve to promote a scientific argument.
Here I point out some selected assumptions made by Johnston and by Goldsmith et al. which I think tend to promote the conclusions they obtain.
Johnston uses a theoretical model of ozone to determine the effect on the ozone layer of nitrogen oxides introduced by hypothetical SSTs. Let me first describe the expected capability of such models. Because of the many complexities of the actual processes involved in determining ozone profiles, any theoretical calculation is necessarily incomplete. For this reason the model is not expected to reproduce or predict all or even very many of the naturally observed features of the distribution of ozone in the stratosphere. All that is attempted is an approximation of a few of the important features of this distribution. For example, in 1971 a good comprehensive model of stratospheric ozone, including chemical reactions and transport processes, might be expected to give average ozone concentrations within 25 to 50 per cent of the observed concentrations, and to give the right sort of seasonal changes (such as an increase in the ozone column in the spring at high latitudes). It would not be expected to reproduce or predict day-to-day fluctuations in ozone, or even to attempt to do this sort of thing. A given ozone model will be expected to reproduce or predict only certain features of ozone distributions, depending on the physical and chemical processes that are incorporated in it. Johnston's model is designed to give an average ozone profile at a given time of the year and at a particular latitude. It is not designed to give daily varying or seasonally varying ozone profiles.
Having considered the inherent limitations of ozone models, let me turn to the technical assumptions involved in the construction and use of Johnston's ozone model. After developing his model, Johnston uses it to obtain a theoretical profile of naturally occurring ozone. He then incorporates in the model a representation of the injections of NOx from a hypothetical fleet of SSTs. Finally he uses the model to obtain new theoretical profiles, which then show a reduction in ozone. An important aspect of the work involves deciding how to incorporate the SST-NOx into the model. In other words, there must be some method for determining how long SST-NOx remains in the stratosphere, and where in the stratosphere it will spend its time. These decisions are examples of what I refer to as technical decisions.
First consider the question of how long SST-NOx remains in the stratosphere. Johnston uses values for the residence time taken from the SCEP report to decide this. Namely, he uses the SCEP figure of 2 years at 20 km altitude and above. Since the SCEP figures are for any gaseous substance (not just NOx), they are based on the assumption that the particles leave the stratosphere by physical movement. If the particles are also removed from the stratosphere via chemical reactions, the residence time would be smaller. Therefore the values of SCEP might be considered slightly high when applied to NOx, since chemical removal of NOx does occur, though not rapidly.
Even ignoring chemical removal, Johnston's choice of 2 years may be considered a slightly generous estimate. SCEP lists the residence time as 6 months at 15 km altitude, and 2 years at 20 km and above. If the SSTs fly just at 20 km, the residence time would seem to be at most 2 years: if they flew lower the estimate of the residence time would be lower, whereas if they flew higher the time would be no higher. But the SCEP values themselves are peculiar as regards this point (see "residence half-life" in the glossary at the end of part I), so it is questionable whether Johnston's choice is generous or not.
Even taking the above points into account, Johnston's adoption of 2 years can certainly be justified when one considers the large uncertainty in published results concerning residence times. For example, one paper cites estimates ranging from 0.8 to 3.5 years, though for the whole stratosphere rather than for 20 km altitude.
My attitude towards Johnston's adoption of a residence time of 2 years is this. There is a range of residence times at 20 km altitude that in 1971 could be justified on the basis of existing studies, and used without serious criticism. In my judgement this range is from 1 to 2 years, with perhaps 18 months as the most likely, and times below 1 year or above 2 years being harder to justify. Johnston's choice of 2 years is quite justifiable and quite possibly correct. But it happens to be towards the upper end of the range of readily justifiable residence times. As a result it helps in a small way to push Johnston's argument.
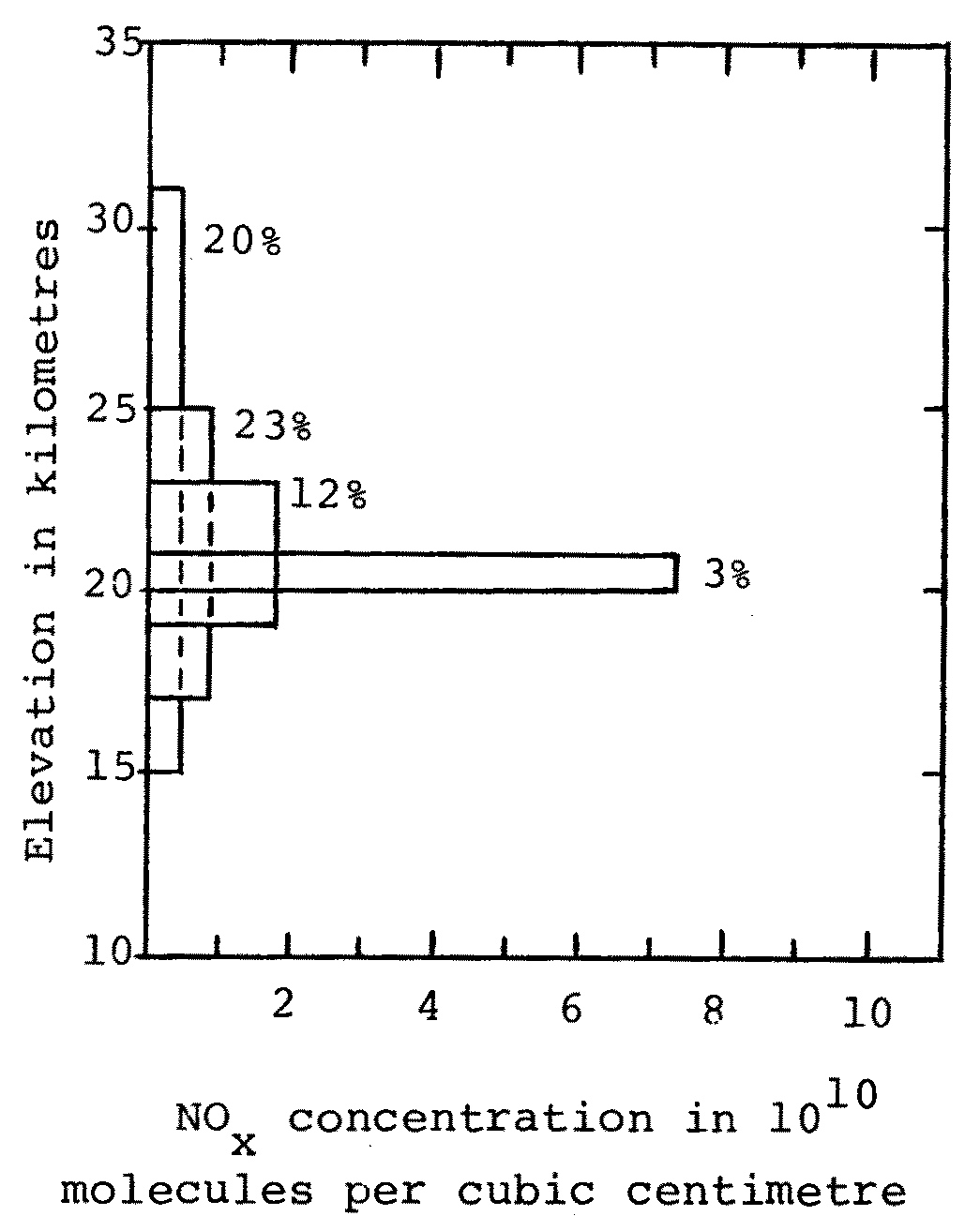
Next consider the question of where the NOx will spend its 2 years in the stratosphere. This question is not easily answered. To determine a reasonable answer one might use data on winds and diffusion rates and chemical removal processes, and numerically solve an appropriate set of equations. This is a fairly complicated and extensive calculational problem, though in principle a straightforward one. To obtain some results without first solving in detail this additional problem, Johnston makes some fairly arbitrary but not unreasonable assumptions. Namely, he assumes that the NOx is evenly spread out over various altitudes. The cruise height of the SSTs is 20 km. Johnston uses models in which the NOx is spread between (A) 20 and 21 km, (B) 19 and 23 km, (C) 17 and 25 km, and (D) 15 and 31 km.
By using several models Johnston presumably hopes to give approximate upper and lower bounds for the likely result of a more rigorous calculation. That is, the result of a rigorous calculation hopefully would give results within the range of results obtained in the series of models. Johnston's four assumed SST-injected NOx distributions, presented in Figure C, give reductions in the ozone column of 3%, 12%, 23% and 20% of the original column. Let me assume for the purposes of illustration that a rigorous and detailed calculation gives the NOx distribution presented in Figure D, and that when this NOx distribution is added to naturally occurring NOx in Johnston's model and the ozone concentrations calculated, a reduction of X% of the original ozone column is obtained. The results of Johnston's model give upper and lower bounds for the result using the distribution from the rigorous calculation if X is in the range from 3 to 23. In addition, the models could be said to be a good or representative choice if X falls near the centre of the range 3 to 23.
It may be readily noticed that the average height for each of Johnston's spreading limits is higher than the cruise height of the SST, namely 20 km. Therefore the assumed NOx distribution resulting from the SST injection would seem to be systematically biased towards higher altitudes. If a set of arbitrary distributions is to be chosen, in which the NOX is spread evenly between certain altitudes, a more natural assumption would seem to be one in which the NOx is systematically spread above and below the SST cruise height. For example, it might be evenly spread between (a) 19 and 21 km, (b) 18 and 22 km, (c) 17 and 23 km, and (d) 15 and 25 km.
Generally NOx in the stratosphere at higher altitudes than the SST cruise height of 20 km has a much greater destructive catalytic effect on ozone than NOx lower than 20 km. Therefore by assuming NOx from SSTs is spread between altitudes on the average higher than 20 km, Johnston apparently pushes his argument that there will be a significant effect due to the SST injections. In fact, his results showing the most spectacular reductions in ozone arise from the assumed distributions between 17 and 25 km and between 15 and 31 km.
Johnston's assumed NOx distributions are not necessarily wrong. There are quite a number of NOx distributions that could be adopted, each with justification. Johnston's assumed distributions can be considered to be based on a particular transport model, which results in more spreading above 20 km than below. (Johnston does not describe such a transport model in his paper, but he has described it to me.) While this transport model is a possible one, there are others which result in different NOx distributions. There were a large number of plausible transport models and NOx distributions which might have been used in Johnston's 1971 calculation. I would say that Johnston's choices of distributions between 17 and 25 km and between 15 and 31 km result in more ozone destruction than would most of these plausible distributions.

Fig. C Distributions of NOx due to a fleet of SSTs operating in the stratosphere, with corresponding reductions in the ozone column, as used by Johnston in his models A through D. The box-like distributions pictured tell where the SST-NOx is assumed by Johnston to be deposited, ranging from a high concentration layer between 20 and 21 km (model A) to a low concentration layer between 15 and 31 km (model D). Note that the percentages in the figure refer to the associated reduction in ozone, as pictured in Johnston's Figure 2, page 521.
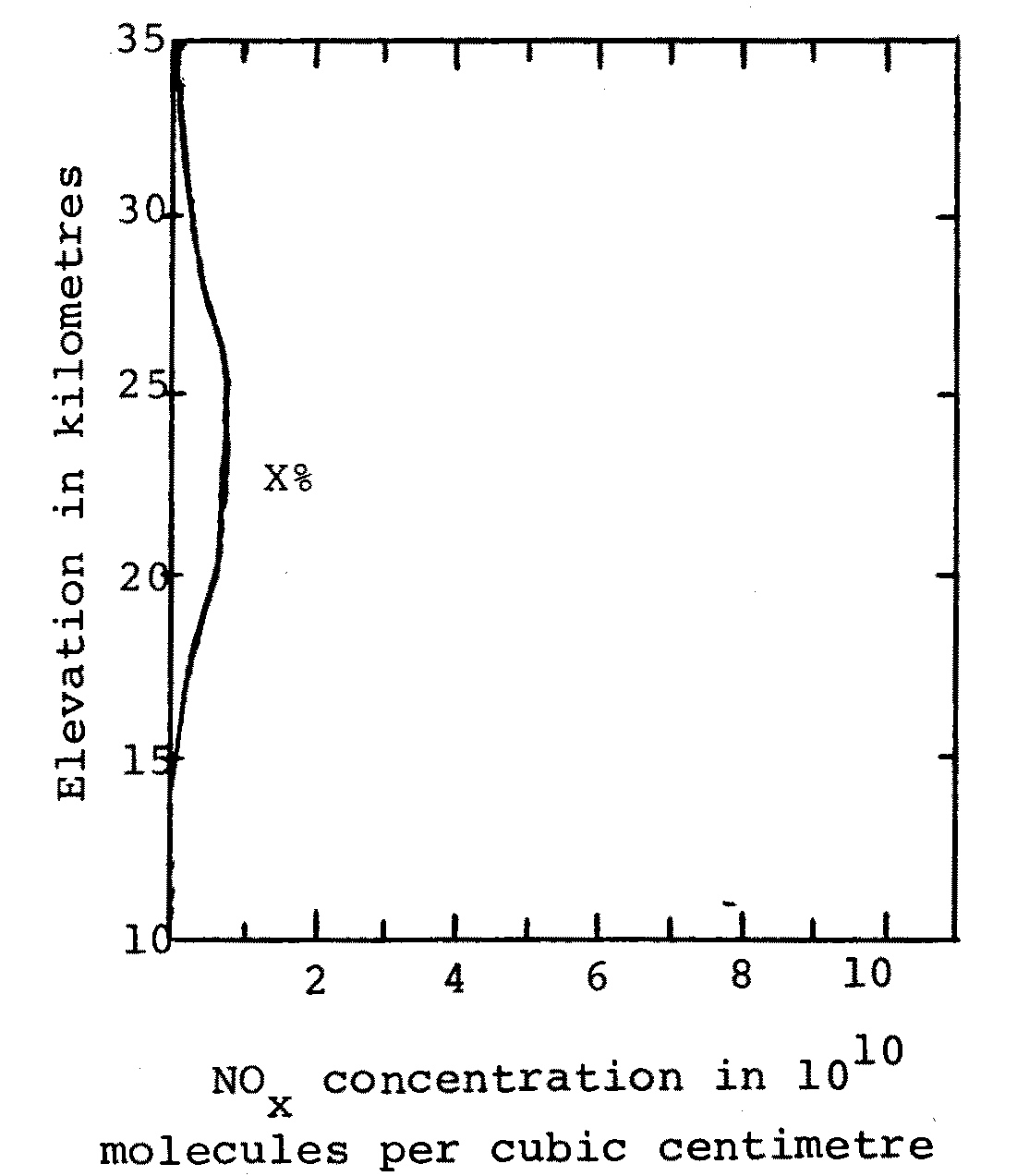
Fig. D A hypothetical realistic distribution of NOx due to a fleet of SSTs operating in the stratosphere, producing X% reduction in the ozone column.
Goldsmith et al. argue that NOx injected into the stratosphere by thermonuclear explosions apparently did not significantly affect stratospheric ozone levels. They further argue that similar amounts of NOx injected by jet aircraft will have no significant effect either. One important technical assumption underlying this argument is that any major effect on stratospheric ozone due to NOx injected into the stratosphere does not depend sensitively on the position, time, or distribution of the NOx injection. To justify this assumption and buttress their arguments, Goldsmith et al. might have determined the space and time distribution of the NOx injected by each method, and determined the expected catalytic destruction of ozone due to the NOx introduced into the stratosphere by each method. A calculation to do this would require extensive and complex calculations. Like Johnston, Goldsmith et al. bypass a detailed consideration of the distribution of NOx in the stratosphere by making a technical assumption. They simply assume that differences in the modes of injection of NOx do not weaken their argument. For example, they state "It is seen that the nuclear testing is equivalent during the period 1952 to 1958 to more than 100 fully operational Concordes." (p. 549, column 1). Their preceding arguments however showed only that the mass of NOx introduced is in each case expected to be the same, and not that the time and space distribution and therefore the expected catalytic destruction of ozone of the NOx is the same.
The aim of Goldsmith et al.'s argument is to show that SST-NOx is not likely to reduce stratospheric ozone. They argue that NOx from stratospheric nuclear tests did not significantly affect ozone levels, and assume that this nuclear test-NOx is at least as effective as SST-NOx in reducing stratospheric ozone. If SST-NOx were in fact found to be equally or less effective in reducing ozone than nuclear test-NOx, their argument holds. But if on the other hand SST-NOx were found to be more effective in reducing ozone than nuclear test-NOx, their argument would be seriously weakened. Without knowing the result of a more detailed calculation, we can only say that Goldsmith et al.'s technical assumption either only sustains their argument or seriously weakens it. Hence in terms of their aim of showing that SST-NOx is not likely to reduce stratospheric ozone, the assumption is more likely to strengthen their case than to weaken it. In the context of what they are trying to demonstrate, their technical assumption can be considered to push their argument.
Next consider an assumption made by Goldsmith et al. in their calculation of the amount of NOx injected into the stratosphere by nuclear explosions. A large part of Goldsmith et al.'s paper is taken up by a description of their method for obtaining an estimate of the amount of NOx produced by a thermonuclear explosion. They do this by picking out a set of elementary chemical reactions thought to be relevant, and solving a set of equations which describe the variation in time of the densities of the chemical species involved. Involved in this method are a large number of technical assumptions, many of which they mention. Examples are: (a) the choice of reactions; (b) the use of pressure-temperature histories for seven shells of gas in which the chemical reactions occur; (c) the neglect of the influence of the energetics of the chemical reactions on the pressure-temperature histories of the shells due to the shock wave; and (d) the validity of the method used to solve the equations.
Here I only discuss one of these assumptions, which I believe tends to push the argument of Goldsmith et al. As the fireball, the hot reacting centre of the nuclear explosion, rises, cools and expands, it takes in a large amount of tropospheric air. This air contains a large amount of water. As the centre of the explosion rises into the stratosphere it cools dramatically [mainly due to expansion]. This causes the water in the air taken in to condense out, and much of it falls to the troposphere as rain. Since NOx is very soluble in water, it might be expected that the eventual amount of NOx injected into the stratosphere would be considerably reduced.
Goldsmith et al. make no mention of this possible effect in their paper. Their only consideration of water is in terms of its possible effect as a reactant in their reaction scheme. By discussing the effect of water in this technical respect, but not mentioning any other effect due to water in the atmosphere, it may be considered that they tend to create the impression that they have considered all important effects of water in their discussion. Their technical assumption is thus that the expected amount of NOx introduced into the stratosphere where it will affect ozone is not significantly less than the expected amount of NOx produced by the thermonuclear explosion.
Goldsmith et al. are certainly aware of this assumption. For example, one of their references is to Johnston, Whitten and Birks, who mention it. Also, in the discussion following the initial conference presentation of Goldsmith et al.'s paper, Johnston raised the point and Goldsmith replied. Goldsmith's reply as printed in the conference proceedings states that although much of the radioactive debris from large thermonuclear tests is water soluble, "we know" (meaning presumably that it is a known fact) that almost all of this radioactive debris reaches the stratosphere. Therefore there is no reason to expect NOx to be rained out. Presumably Goldsmith et al. did not think the point was of sufficient merit to warrant inclusion of even a refutation of Johnston et al.'s point in their paper in Nature. It is apparent that the choice of what constitutes an important or valid argument, worthy of discussion in a published paper, can serve to push an argument.
Once again, Goldsmith et al.'s technical assumption about the lack of any significant raining out of nuclear test-NOx is both justifiable and possibly correct. But if one is making an assumption about this matter, the one chosen by Goldsmith et al. is precisely the one which serves to promote their argument to the greatest extent. Any other technical assumption, such as that 20% of the nuclear test-NOx is rained out, would weaken their conclusion. For this perspective the technical assumption serves to push their argument.
In their papers Johnston and Goldsmith et al. make a large number of technical assumptions. By examining some of these assumptions, I have tried to illustrate how it is possible for a scientist to push a scientific argument by the choice of technical assumptions.
To push an argument by the choice of technical assumptions does not mean that the assumptions are either unjustifiable or necessarily wrong. Often there are quite a few different assumptions that can be made, all of which can be justified, and any one of which may turn out to be correct. Whether or not they present justifications in their papers, this is the case for the assumptions of Johnston and of Goldsmith et al. which I have analysed. But out of plausible and justifiable assumptions that could be made, I suggest that Johnston and Goldsmith et al. chose assumptions that promote their conclusions more than would the majority of the assumptions they could make.
Johnston's and Goldsmith et al.'s papers are written for a purpose. Johnston is drawing attention to an effect which he believes is important, and Goldsmith et al. are trying to show that this same effect is not significant. It is in the context of what they are attempting to do that the technical assumptions of these scientists serve to push their arguments. The majority of other technical assumptions that they could reasonably make would not promote their arguments to the same extent as the ones they actually choose.
This is not to say that technical assumptions are always chosen so as to give the maximum possible support for a conclusion. Many times a technical assumption may weaken a conclusion or be of no consequence to it. I only suggest that pushing via technical assumptions is not an unusual occurrence.
In one way pushing of scientific arguments through the choice of technical assumptions seems virtually inevitable. To be published and noticed and studied, scientific work needs to be scientifically interesting. It needs to reach a recognisable and perhaps dramatic conclusion, which can be followed up or challenged in future work. Significant scientific work is almost always very meaningful to other scientists. But meaningfulness is not easily achieved unless there is a definite result or conclusion of some kind. A research paper that concludes, "maybe this, maybe that, maybe the other, but we really can't say much" is unlikely to get published or read.
Some technical assumptions may lead to scientifically interesting conclusions, while others lead to inconclusive results. I suggest that scientists learn to adopt those types of technical assumptions that will lead them to the scientifically interesting conclusions which they are looking for. In this way pushing of the argument through the choice of technical assumptions may be built into the conventional way in which science is practised. From this point of view, the pushing of the argument through choice of technical assumptions by Johnston and by Goldsmith et al. can be seen as a natural feature of their production of scientifically interesting research.
Some of the technical assumptions made by Johnston and by Goldsmith et al. that I have not considered involve:
(1) Neglect in calculations of further chemical reactions that may be of some importance. A number of different workers since Johnston's 1971 paper have included many further reactions - such as the ones listed by Johnston but not included in his calculations - in their calculations. See the review of studies in Grobecker et al. (1974).
(2) Lack of consideration of the influence of changes in ozone levels on stratospheric temperatures. Inclusion of this effect leads to smaller ozone decreases for a given amount of added NOx. The effect is analysed by Blake and Lindzen (1973). McElroy et al. (1974) compare ozone reductions with and without induced temperature changes.
(3) Lack of consideration of density inhomogeneities (for example in NOx from SSTs) on creation and destruction rates of ozone. This effect is analysed in the abstract by Donaldson and Hilst (1972); these workers have applied it to the SST-NOx problem in various conference presentations.
(1) Neglect of the effect of energy absorbed in the creation of nitrogen oxides in a nuclear blast on the temperature and pressure history of the blast.
(2) Lack of a rigorous statistical analysis to show what effect on ozone from nuclear test-NOx could be expected from an inspection of ozone records.
The assumptions concerning these latter two points are attacked and defended in the "discussion" following Goldsmith et al. (1973a). Johnston et al. (1973) present an alternative analysis of ozone records. Johnston (1974, 1974a) briefly discusses Goldsmith et al.'s assumptions.
The complexity of modern ozone models, and their limited ability to reproduce or predict ozone distributions, are illustrated by the models and results of London and Park (1974), McElroy et al. (1974), and Cunnold et al. (1975).
My judgement that justifiable residence times in 1971 at 20 km might range from 1 to 2 years is based on my study of work in the area performed both before and since 1971. The stratospheric residence times surveyed by Pressman and Warneck (1970), and which range from 0.8 to 3.5 years, and most of which are less than 2 years, are for the whole stratosphere. One might expect times at 20 km to be rather less. Therefore I believe the SCEP value at 20 km to be, if anything, an overestimate.
The argument that much NOx produced in nuclear explosions may be rained out is presented by Johnston et al. (1973, p. 6110). This argument was also included in the earlier version of this paper (Johnston et al., 1973a), which is referred to by Goldsmith et al. Johnston and Goldsmith present their respective views in the discussion following the conference version of Goldsmith et al.'s paper (Goldsmith et al., 1973a).
A scientist in developing an argument to support an hypothesis draws evidence from a number of sources. In presenting evidence one must always be selective - all the evidence and arguments cannot be presented. Often different authorities support different viewpoints, present different 'facts', and offer different interpretations of evidence. Depending on the field, a scientist may draw sound support for many points of view and find some support for nearly any view. Therefore it is easy for a scientist, knowingly or unknowingly, to push an argument by selective choice and use of available evidence.
Johnston uses the conclusions of the SCEP report as a basis for development of his argument that SST-emitted NOx could seriously reduce stratospheric ozone. The authors of the SCEP report make a number of assumptions concerning the possible problem of NOx emissions. Among other things, these assumptions cover: (1) the level of emissions from SST exhaust; (2) the average amount of time spent by the exhaust particles in the stratosphere; and (3) the concentration of exhaust particles to be expected at different places in the stratosphere. At the time the SCEP report was produced, each of these assumptions was more or less of a guess; that is, the uncertainty associated with each estimated value is large. For example, the SCEP report authors note that estimates of the average time between evaporation of a carbon dioxide molecule from the surface of the ocean and its subsequent reabsorption on the surface range from 3 to 50 years. This range of values also approximately indicates the degree of uncertainty associated with the levels of SST-NOx emissions and the expected stratospheric concentrations of exhaust particles. Given these large uncertainties, the choosing of one particular value rather than another can lead to sizable differences in results.
For the level of emissions from SST exhaust, Johnston picks a value typical of the SCEP report. For the estimated amount of time spent by the emitted particles in the stratosphere, he uses consistently the SCEP value of 2 years. But for the SCEP assumption concerning the expected increase in the concentrations of emitted particles to be found in different regions of the stratosphere, Johnston alters the SCEP report assumptions. This aspect of the problem is worth considering in more detail.
Given that a fleet of SSTs emits exhaust particles into the stratosphere at a certain rate, and that these particles spend on average a certain amount of time in the stratosphere, then one can easily calculate the total number of particles in the stratosphere due to continued operation of the SST fleet. Not so easy to calculate is where the particles will spend their time - that is, the concentration of the exhaust particles in different parts of the stratosphere. The SCEP report authors made very rough estimates based on the following reasoning. Consider a region of high concentration, typically near heavily travelled regions. The absolute minimum concentration of the particles at such a point would occur if the exhaust were evenly spread over all the stratosphere. This minimum level was calculated by the SCEP report authors. Then to account for the fact that the concentration would certainly be higher at some points than at others, they estimated that the highest concentrations at heavily travelled regions would be up to ten times this minimum value. Note therefore that the "minimum" figure and the figure 10 times as high both refer to a region of heavy SST travel. The SCEP report authors in this way hope to obtain lower and upper limits for the concentration in a region of heavy SST travel. For regions far from SST operations, the concentration would be smaller than either the minimum or maximum concentrations in a region of heavy SST travel.
So that you can see exactly what is involved, the relevant section of the SCEP report is reprinted at the end of this chapter. SCEP's "world average concentration" is their estimate of the minimum concentration in a region of heavy SST travel, and their "possible peak concentration" is their estimate of the maximum concentration in a region of heavy SST travel.
The SCEP report authors do not consider the distribution of the injected contaminants in more detail than this. Johnston, however, makes more specific assumptions as to the location of the SST-emitted NOx since a detailed specification is necessary to enable his calculations to be made. He assumes that the SST-NOx is spread over certain altitudes only, for example from 19 to 23 km, as discussed in the previous chapter. Now the important point is that the assumption that NOx is spread only over certain altitudes effectively increases the estimated concentration at those altitudes where the NOx is spread, making it larger than the minimum value noted in the SCEP report. This is perfectly consistent with the picture in the SCEP report: the 10-times factor was meant to take into account such localisation. However then in addition Johnston applies the SCEP factor of 10 to his localised distributions to get values over highly travelled regions, thereby obtaining NOx concentrations more than 10 times the estimated global background, and indeed more than the estimates of peak concentrations made by SCEP. Johnston says "the SCEP report accepted as harmless mole fractions of 6.8 x 10-9 and 6.8 x 10-8" (page 517, column 2). Johnston's mole fractions, in the regions where he assumes the SST-NOx to reside, are considerably larger than this.
The mole fractions assumed by Johnston for his 8 different cases (p. 521) are approximately:
A 4.5 x 10-8
B 1.2 x 10-8
C 5.8 x 10-9
D 3.3 x 10-9
E 4.5 x 10-7
F 1.2 x 10-7
G 5.8 x 10-8
H 3.3 x 10-8
Note that the mole fractions for cases E and F are larger than 6.8 x 10-8, the largest value given by SCEP, and the mole fractions for all but cases C and D are larger than the minimum value (in a high traffic region) given by SCEP.
This is a good point to interrupt and illustrate how my argument may be pushed. Johnston noted to me that instead of writing just the mole fractions for his cases A to H, these might be supplemented by the associated ozone reductions obtained
using his model:
A 4.5 x 10-8, 3%
B 1.2 x 10-8, 12%
C 5.8 x 10-9, 23%
D 3.3 x 10-9, 20%
E 4.5 x 10-7, 3%
F 1.2 x 10-7, 14%
G 5.8 x 10-8, 42%
H 3.3 x 10-8, 50%
Just writing the mole fractions might suggest that the greatest reductions in ozone obtained by Johnston are for cases in which the mole fractions are larger than those in the SCEP report. The inclusion of corresponding ozone reductions shows that this is not true. The greatest reductions in ozone obtained by Johnston are for cases C, D, G, and H, for which the NOx mole fractions are less than the SCEP mole fractions. By omitting the ozone reduction percentages I might suggest the incorrect idea that Johnston obtained his largest ozone reduction percentages with NOx mole fractions larger than the SCEP values, and thereby push my argument that Johnston is pushing his argument.
Nevertheless, I would still argue that Johnston pushes his argument through his use of the 10-times factor. For while his mole fractions for cases C, D, G, and H are less than the SCEP mole fractions, they are not less than the SCEP values reduced by a factor 0.35, which Johnston says he is using. Certainly Johnston's use of the factor of 10 as well as assumptions concerning the details of the spatial distribution of NOx, thereby increasing the local concentrations, is not in keeping with the spirit of the SCEP assumptions which Johnston otherwise accepts.
The most obvious selection of evidence by Goldsmith et al. is that of ozone records. After choosing certain ozone records, they search these records for evidence of a reduction in ozone due to thermonuclear explosions. Therefore it would seem natural that Goldsmith et al. should choose the records according to their appropriateness for a study of this kind.
Goldsmith et al. use data only from 17 ozone measuring stations, selected according to certain rather restrictive criteria (given on page 549, column 2 of their paper). These criteria apparently have nothing to do with the usefulness of the records in showing that there was or was not any observed reduction in ozone correlated with the nuclear weapons tests. For example, one of the criteria is that the records "do not have gaps of more than 18 consecutive months in their data; and they have more than 110 monthly mean values for the total period 1957 to 1970." It is not obvious why records not satisfying this criterion should be excluded, as long as the data is given an importance according to the number, locations, time and quality of the measurements made. Such an exclusion would seem justified only if having a gap of more than 18 months in the data contributes to the records being of no use whatsoever as an indicator of changes in the ozone level which might result from injections of NOx from nuclear explosions. The same sort of comment applies to the other criteria used.
In short, the ozone records used by Goldsmith et al. were not chosen originally solely for their suitability for the problem of detecting changes in total ozone.
The importance when selecting ozone records of using criteria which are appropriate for the task at hand is emphasised by Pittock. He notes that because stations measuring ozone tend to be clustered at particular latitudes, especially in the middle latitudes of the northern hemisphere, averaging of all available measurements does not give one a good idea of world trends in total ozone. To get these more accurately it is necessary to give more than average significance to data from those stations in regions of the world where there are few stations. The goal of Goldsmith et al. is to observe the effect of NOx from nuclear weapons tests upon stratospheric ozone. An analogous sort of procedure, giving different stations different degrees of importance, would seem to be appropriate for Goldsmith et al.'s purposes.
The result of Goldsmith et al.'s procedure is that they use a smaller amount of data than they might have used. Therefore the confidence one can place in their results must be reduced. Goldsmith et al.'s results would be much more convincing if the results were shown to be reasonably independent of the specific ozone records chosen. On the other hand, if other researchers were to choose a different but equally plausible set of records, and were to obtain a different conclusion, then the confidence in Goldsmith et al.'s results would be much reduced. And this is exactly what happened.
Goldsmith et al. consider a counterargument of Johnston, Whitten and Birks. Let me describe that counterargument briefly. Johnston et al. evaluate a different set of ozone records than do Goldsmith et al. They use all the data for ozone columns in the published volumes of Ozone data for the world. They give the data for a given month and place an importance in their calculations proportional to the number of observations made that month and at that place. Also they give the data for a given station an importance inversely proportional to the average spread of the day-to-day variations in that station's data, and in this way give more importance to data that is more consistent. Relevant to Pittock's point that ozone trends over the world are not given by the average of all available measurements, Johnston et al. note that "The averages we give may not be interpreted as 'global averages'; rather they are 'weighted averages' of all the stations in the world." In any case, Johnston et al. conclude that a possible effect on ozone correlated with the nuclear weapons testing cannot be discounted.
Goldsmith et al. do not consider the effect that a difference in the choice of ozone records could have on their conclusions. For example, they refer to Johnston et al.'s conclusion, which is that the decrease in ozone during 1960-1962 and the increase from 1963-1970 might be ascribable (perhaps only in part) to reduction in ozone due to nuclear weapons tests and subsequent recovery from the effects of the test period. However in referring to this conclusion, Goldsmith et al. refer (apparently) to their own data: "The ozone data going back to late 1957 given in Fig. 3, do not support the contention" (page 550, column 1). Thus they seem to pre-empt for themselves the status of using "the" ozone records, as if these records were not a special selection. They seem to exclude a choice of data such as Johnston et al.'s as being suitable for the purpose at hand, without showing that their own criteria are more suitable.
Thus concerning the possible effects on stratospheric ozone due to nuclear weapons tests they say "It seems to us that these records do not provide evidence for such a modification" (page 550, column 1). But they do not consider how the effect of the choice of the records, as evidence, affects their conclusion.
Goldsmith et al. do discuss the difference between their choice of ozone records and Johnston et al.'s choice in the following way. After noting their own criteria of excluding measurements made with the Russian filter ozonometer and those in the southern hemisphere, they note that Johnston et al.'s analysis included ozone measuring stations in the Soviet Union and some in the southern hemisphere. But this mention of some of the stations used by Johnston et al. is irrelevant to the question of deciding which choice is more useful for determining reductions in ozone due to nuclear test-NOx. Given Goldsmith et al.'s criteria, it says nothing more than that Johnston et al. used some stations that they did not. But the likely effect on the reader of Goldsmith et al.'s discussion on this matter is to suggest that Goldsmith et al.'s choice of ozone records is more suitable than Johnston et al.'s
Another interesting selection of evidence is Goldsmith et al.'s presentation of the long term records of Arosa and Oxford in considerable detail. From the way this data is presented it is difficult at best to get any general picture of general ozone changes, since the data points are scattered across the graph. Even if a change were observable, it would not be statistically significant because of the large scatter and limited number of stations (namely two). Yet this extra analysis and presentation of data give the appearance of strengthening Goldsmith et al.'s case, although nothing relevant to the hypothesis is newly added.
The appearance of strengthening the argument arises from at least two sources. The first is the line of reasoning that if Goldsmith et al. went to the trouble to include these results, and they are not obviously a source of difficulty to their argument, then one might reasonably assume that they must support it. In other words, most readers assume that material introduced in a paper is relevant. Second, Goldsmith et al. conclude, immediately after their discussion of this data, that "In common with other data presented here, there is nothing to suggest that nitrogen oxides from the testing of nuclear weapons in the atmosphere have had any effect on the total ozone." (page 550, column 2). This is, they imply that the data from the two stations supports their conclusion.
Another reason given for the inclusion of the Arosa and Oxford data is that it demonstrates the high variability in total ozone, and indicates the difficulty in detecting long term trends. Actually this would appear just as well to be an argument for not drawing a strong conclusion as to the absence of any looked for effect on ozone. Goldsmith et al. thus use the evidence for their own purpose when it might be thought to be irrelevant or even supportive of an alternative hypothesis.
Related though different from selective use of evidence is selective consideration of uncertainties. Associated with any scientific result there is a greater or lesser degree of uncertainty (a probability of error or deviance from the result later considered to be correct by the scientific community.) One of the tasks considered most important in the presentation of scientific results is a discussion of possible sources of error and the extent of their influence on the validity of the results obtained. By selective consideration and emphasis on different sources of uncertainty it is possible for a scientific argument to be pushed. Here I only summarise the conclusions from a more detailed analysis of selective consideration of uncertainties in the work of Johnston and of Goldsmith et al.
Johnston treats the different uncertainties in his argument in different ways. He emphasises tests of some of the uncertainties in his model. For example, the effect of uncertainties in parameter values is studied using sensitivity analysis. The uncertainties which are so tested happen to be those which do not significantly affect his result. On the other hand, he does not emphasise uncertainties which cannot be easily tested, such as lack of ozone transport in the model. Finally, he usually does not calculate a range of results when uncertainties might be considered to warrant this, as in the case of the amount of NOx which is injected into the stratosphere. The one case in which he does calculate a range of results, namely concerning where NOx spends its time in the stratosphere, is the case in which he by other means pushes his argument.
In Goldsmith et al.'s paper there are uncertainties associated with their calculation and assumptions in each of the four major components of their argument: calculation of the amount of NOx produced by nuclear tests, calculation of the amount of NOx produced by Concorde, equivalence of equal amounts of NOx from nuclear tests and from Concorde for affecting ozone, and demonstration of a negligible effect on ozone due to nuclear test-NOx. The uncertainties are discussed in detail only in the first of these components, and even then the discussion is inadequate and unbalanced. For the other three components there is little mention of the uncertainties. If full consideration were given to uncertainties in all the four areas, Goldsmith et al.'s conclusion would lose much of its persuasiveness.
In short, Johnston and Goldsmith et al. may be considered to be pushing their arguments by emphasising small uncertainties which do not affect their conclusions, and de-emphasising or ignoring uncertainties which call their conclusions into question.
Evidence is never cut-and-dried or neutral. First, evidence must be selected. By choosing certain evidence out of the total available evidence, it is inevitable that certain viewpoints and conclusions will be favoured. Second, evidence must be interpreted. The significance of the evidence depends on the context in which it is used: on the concepts which are used to understand it, on the theoretical framework in which it is applied, and on its relevance to what are perceived to be significant problems. Finally, evidence is used in a certain way and for certain purposes. Depending on these ways and purposes, the evidence may have a variety of different effects on a scientific argument and conclusion.
By selecting, interpreting, and using evidence for certain purposes it is possible for a scientist to push a scientific argument. Goldsmith et al. select only certain ozone records as being important for their purposes. Johnston interprets the SCEP 10-times factor in a way that makes his results more significant than they would be otherwise. Goldsmith et al. use evidence about variability in ozone levels to support rather than weaken their conclusion. I believe that Johnston and Goldsmith et al., like most scientists most of the time, consistently select, interpret and use evidence in a way that selectively promotes their arguments and conclusions.
Pittock (1972) contains the analysis of ozone record selection. The description of Johnston et al.'s method of pooling data, and the quote, are on page 6120 of their 1973 paper.
Excerpt from Study of Critical Environmental Problems (SCEP), Man's impact on the global environment (Cambridge, Massachusetts: MIT Press, 1970), pp. 71-74.
The emissions from a single GE-4 engine to be used on the B2707-300, flying at 65,000 feet (about 20 km) in a cruise mode, at Mach 2.7, on a day with standard weather conditions, have been calculated (Table 1.4), assuming chemical equilibrium, by General Electric Company engineers (Hession, 1970; Thompson, 1970). In the future, the calculations will be compared with actual engine tests, but it is estimated by SST officials (Thompson, 1970) that the fraction of products per unit amount of consumed fuel may be accurate within 10 percent (except as indicated later). It should be noted that the engine will be equipped with an afterburner; however, it will be in only partial operation in the stratosphere, as opposed to full operation during takeoff; that is, the temperature in the afterburner will be 2,800oR (degrees Reaumur) while cruising in the stratosphere, but will be 3,500oR during takeoff.
An estimate suggested by the federal Aviation Administration (FAA) calls for 500 SST aircraft to be flying during the period 1985-1990. Each of these jets would fly in the stratosphere for almost 7 hours a day (2,500 hours per year). Of the 500 jets, 334 would be U.S. and be equipped with four engines, and 166 would be of non-U.S. fabrication and would have the equivalent of two engines.
Tables 1.5 and 1.6 contain our estimates of equilibrium or steady-state concentrations, after several years of operation, assuming a 2-year mean residence time for all products.
Conclusions
The following conclusions are based on the assumed operation of 500 commercial SSTs in the period 1985-1990, and on their emissions as provided by the federal SST office (except as noted).
1. Because of the long atmospheric residence time of CO2 and the relatively small CO2 contribution from SSTs compared with that from other sources of fossil fuel combustion, there will be no special CO2 problem due to SST operations.
2. Stratospheric water vapor will increase, on a global average, by 0.2 ppm by mass (from 3.0 to 3.2 ppm). Since there will be more water vapor added to the north temperate latitudes, parts of this region may perhaps have a contribution to standing concentration as much as tenfold higher than the increase of the global average (that is, grow from 3 to 5 ppm of water vapor).
3. Concentrations (by mass) of CO, NOx, SO2, HC, and soot will range from fractions of a ppb for soot to 68 ppb for CO and NO from 500 SSTs in the north temperate latitude, peak of SST activity. Emissions from present large engines are appreciably different from those predicted for the SST GE-4 engines. The current NO emission rates per gallon of fuel for certain jet engines are as much as twentyfold lower, but the HC can be fourfold greater and the particles more than tenfold greater. Fuel with a 0.3 percent sulfur content (the maximum allowable limit) would increase the emission of SO2 by more than sixfold over our SST estimates, and use of 0.01 percent sulfur fuel (technically feasible) would reduce the SO2 emission to one-fifth of our estimates, since the calculations here are based on 0.05 percent sulfur content. Further, there are doubts concerning the applicability of particle emission information derived from both theoretical calculations and static field tests to the real atmosphere and operating conditions. It is felt that realistic operation may produce larger numbers of particles. Finally, we have no information on fuel additives.
Table 1.4 |
|
|---|---|
| Constituent | Pounds per Hour |
| Ingested air and consumed fuel | |
| air | 1,380,000 |
| fuel | 33,000 |
| Unused air | |
| N2 | 1,039,000 |
| O2 | 208,000 |
| Ar | 19,300 |
| Combustion products | |
| CO2 | 103,500 |
| H2O | 41,400 |
| CO | 1,400 |
| NO (a) | 1,400 |
| SO2 (b) | 33 |
| Soot (Particles) | 5 |
| Unused fuel | |
| Hydrocarbons (c) | 16.5 |
(a) The General Electric Company advises (Thompson, 1970) that the true NO output is likely to be no more than one-half to one-third of the calculated value. A few past comparisons suggest that measured values will be values that are 10 to 15 percent of the calculated numbers (Thompson, 1970).
(b) Sulfur content of SST fuel will be specified as no more than 0.3 percent sulfur by weight. The 33 pounds per hour given in the table corresponds to 0.05 percent sulfur by weight, which is the average sulfur content of currently available jet fuel, as determined by the Department of the Interior. An appreciable amount of jet fuel with less than 0.016 percent S is available now, according to a Boeing Company survey (Swihart, 1970). A leading producer has told us that it is technologically feasible to produce jet fuel with only 0.01 percent S, but that present production facilities are not adequate to supply a large market.
(c) The General Electric Company expects the true hydrocarbon emission to be vanishingly small, due to the high temperature of the afterburner (Thompson, 1970).
| Table 1.5 Gaseous Concentrations |
|||
|---|---|---|---|
1011 Grams/Yr (a) |
World Average Concentration (b) |
Possible Peak Concentrations (c) |
|
| CO2 | 1,960. |
Not relevant |
|
| H2O | 783. |
0.20 ppm (0.3) |
2.0 ppm (3.0) |
| CO | 26.3 |
6.8 ppb (7.3) |
0.068 ppm (.073) |
| NO | 26.3 |
6.8 ppb (7.3) |
0.068 ppm (.073) |
| SO2 | 0.63 |
0.16 ppb (0.20) |
1.6 ppb (2.0) |
| Hydrocarbon | 0.31 |
0.081 ppb (0.087) |
0.81 (0.87) |
(a) This column is computed using data from Table 1.4 and assumptions for full-scale SST operations for 1985-1990 given in the text.
(b) This column is computed using the data from the first column and the assumption of a two-year mean residence time for all products in this portion of the stratosphere. The mass of the atmosphere is taken as 5.14 x 1021 grams, with the stratosphere containing 15 percent of that, or 0.77 x 1021 grams. The numbers in parentheses represent rounded values obtained by increasing the SST-produced concentration to accommodate subjective estimates of military injections of contaminants into the stratosphere. All concentrations are by mass.
(c) The last column is obtained by multiplying the world average by 10 and represents a tentative upper limit for certain temperature change considerations that would apply to a region of high SST activity.
| Table 1.6 Particle Concentrations (.0001 ppm by mass) | ||||
|---|---|---|---|---|
Formed from SST Exhaust |
Measured at SST Altitude |
|||
Global Average (a) |
Peak N. Hem. (b) |
Pre-Agung (c) |
Post-Agung (d) |
|
| SO4- | 2.4 (4.8) (e) |
24 (48) |
1.2 |
36 |
| Hydrocarbon | 0.81 (1.6) |
8.1 (16) |
- |
- |
| Soot | 0.25 (0.5) |
2.5 (5) |
- |
- |
| Total | 3.46 (6.9) |
34.6 (69) |
1.2 |
36 |
(a) The SO4- figure was calculated using data in Table 1.5 and assuming all SO2 is converted to SO4- (Note that SO4- is heavier than SO2-) The hydrocarbon figure is taken from Table 1.5 and assumes total conversion of gas to particles. The soot figure is computed using data from Table 1.4 and assumptions for full-scale SST operations for 1985-1990 given in the text.
(b) A factor of 10 is applied to the first column in order to estimate the peak concentration within the nonuniform distribution over the globe and would apply to the regions, mostly in the Northern Hemisphere, of large SST activity.
(c) Source: Junge, 1963. No data exist for carbon or hydrocarbon concentrations in the stratosphere.
(d) Source: Cadle et al., 1970
(e) Numbers in ( ) are twice the calculated value, to account for particle settling. It is believed that sulfate particles, because of their slow settling speeds, remain concentrated in the sulfate layer rather than disperse throughout the vertical extent of the atmosphere. The natural sulfate layer happens to be at about the same altitude as the SST injections. The net effect is probably an increase by about a factor of 2 over the concentration calculated for a gas, which would diffuse upward as well as downward. This factor of 2 has been applied in Table 1.6 (the values in parentheses) to the calculated concentrations of all three particles - sulfates, hydrocarbons (HC), and soot - for the same reason.
Cadle R. D., Lazrus, A. L., Pollock, W. H., and Shedlovsky, J. P., 1970. The chemical composition of aerosol particles in the tropical stratosphere, Proceedings of the American Meteorological Society Symposium on Tropical Meteorology (unpublished).
Hession, J. P., 1970 (private communication to A. K. Forney).
Junge, C. E., 1963. Air chemistry and radioactivity (New York: Academic Press).
Swihart, J., 1970. Results of survey by the Boeing Company of sulfur content of present jet fuels (private communication to W. W. Kellogg).
Thompson, J., 1970. Information on the calculated emissions of the GE-4 engine (private communication to L. Machta).
One of the most effective ways to push one's argument is to selectively use only or mostly those results which favour one's own case, to ignore contradictory findings and to drop qualifying statements. This device is used thoroughly and consistently in the papers by Johnston and by Goldsmith et al. I shall only point out the most striking instances of this procedure: the difference between the qualified conclusion arrived at in the texts of the papers, and the statements of conclusions found in the abstracts and summaries.
Johnston in his paper presents a theoretical model for stratospheric ozone: with each given set of relevant data for reactions, rate coefficients, concentrations of chemical species in the stratosphere, and the like, he calculates an ozone profile. The profile constitutes a representation (albeit a crude one) of the actual distribution of ozone in the stratosphere. Johnston then incorporates into the model certain amounts of NOx which he expects might result from operation of a fleet of SSTs, and recalculates the ozone profile. The results are sensitive to where this added NOx is assumed to be. To avoid being too arbitrary, Johnston assumes 4 regions over which the NOx is assumed to be spread, as discussed in chapter 1. On recalculating the ozone column for each of these assumed regions of NOx injection, the original ozone column is found to be reduced by 3%, 12%, 23% or 20%. If the injections of NOx are increased 10-fold, the reductions in the ozone column are found to be instead 3%, 14%, 42% or 50%.
Johnston reports that similar results are obtained when different values of the parameters in the model are used in making his calculations. The reductions he presents in the Science paper are relative to the "standard" model which he uses as best representing stratospheric ozone. Johnston notes that he obtains similar results when the NOx additions are made to variants of the standard model. Also, he refers to further calculations with altered temperatures, different solar angles, and other changes in model parameters. In each case the calculated change in ozone due to SST-NOx is about the same (p. 521, column 3).
Johnston obtains then a virtual multitude of results. Out of these he chooses 8 specific results to present. One would expect that the 8 particular results are chosen because they are obtained using the most appropriate experimental values which are used in the model. This is the case for Johnston's choice of values of parameters such as temperature and naturally occurring NOx. Johnston finds that certain values of the parameters in his model give the best representation of the observed ozone distribution, and uses these values when obtaining his 8 results that are presented in the paper.
One would also expect that the 8 results presented are representative of results obtained using a wide range of plausible assumptions. That is, using models of ozone with altered physical assumptions (such as using a constant solar angle) or different data (such as different temperatures), one would expect to find the percentage changes due to SST-NOx to be sometimes more and sometimes less than the changes obtained using the standard model. Again this is the case. For example, Johnston uses 11 assumed non-uniform distributions of naturally occurring NOx. He finds reductions of ozone for case H (SST-NOx spread between 15 and 31 km, multiplied by 10) for each of these 11 distributions to be in the range 48% to 52%. In this respect the particular naturally occurring NOx distribution presented in the Science paper gives representative results.
In summary, out of a wide range of results from his theoretical model, Johnston presents results for 8 specific calculations, giving reductions of ozone to 97%, 88%, 77%, 80%, 97%, 86%, 58%, and 50% of its original level, due to NOx from an SST fleet. In the previous chapter I noted the special assumptions surrounding the latter four figures. But otherwise these 8 figures seem to be reasonably representative of results from a range of models - models with different values for parameters and models incorporating different physical assumptions. Yet it is the most drastic reduction figure of the 8, 50%, that appears prominently in Johnston's abstract and summaries.
One of the two sentences in Johnston's abstract reads, "The projected increase in stratospheric oxides of nitrogen could reduce the ozone shield by about a factor of 2, ...". In the concluding paragraph of the paper there is the sentence, "The purpose of this report is to point out that if concentrations of NO and NOx are increased in the stratosphere by the amounts accepted by the SCEP report and by governmental agencies, then there would be a major reduction in the O3 shield (by about a factor of 2 even when allowance is made for less NOx emission than SCEP used)." The sentence from the abstract uses the word "projected", which must refer to the SCEP estimates for NOx injected by SSTs as modified by Johnston and as assumed by Johnston to be distributed in the stratosphere. Note also that the first part of the quoted sentence from the concluding paragraph again does not mention Johnston's assumed distributions of NOx. Johnston's argument that SST-emitted NOx will have a significant influence on stratospheric ozone is thus promoted by selective quotation of his own most significant result, without the qualification that this is the most extreme of a set of results.
That the model selected as standard gives for a maximum reduction the figure of 50% (rather than 49% or 51%, say) may be considered fortuitous. Whatever the reason, the figure of 50% is delightfully easy to remember. It is not surprising that when featured in the abstract and summary this figure becomes in the minds of readers the result of Johnston's calculations.
In the body of their paper Goldsmith et al. describe their detailed calculation which leads to an estimate for the NOx production resulting from nuclear tests. They also make assumptions as to the amounts of NOx emitted by a normally operating Concorde. Comparing the figures obtained, they determine the estimated number of Concordes required to produce the same amount of NOx per year as calculated to be produced by the given series of nuclear weapons tests in that year. For example, they determine that according to their calculations of Concorde NOx emission rates and of nuclear weapons NOx production, 1047 Concordes would have been required flying 10 and 2/3 hours per day to produce the calculated amount of NOx produced by nuclear explosions in the peak year of 1962.
Goldsmith et al. point out that the forms of the injection of NOx into the stratosphere by Concorde and by nuclear tests may not be the same meteorologically. That is, the altitudes, latitudes, and times of the year, and the incremental concentrations involved in these two forms of injection are not the same. The difference is important since the effects of a given amount of NOx on ozone depend strongly on where, when, and in what amount it occurs in the stratosphere. In the body of their paper there are a number of qualifications about the accuracy of the estimates of the injections, and about the difference in the forms of the injections. In spite of this, in several places they flatly say that these injections were equivalent. For example, "It is seen that the nuclear testing is equivalent during the period 1952 to 1958 to more than 100 fully operational Concordes." (page 549, column 1). Later in the concluding "Implications" section they state, "We conclude that past nuclear explosions have been equivalent, as far as nitrogen oxide stratospheric injections are concerned, to large numbers of these fully operational Concordes." (page 550, column 2). In the context of the paper as a whole "equivalent" means equal amounts. Goldsmith et al. realise and acknowledge that equal amounts of NOX do not necessarily have equal effects on ozone. However, the statements quoted do not seem to imply this. Omitting qualifications in these statements constitutes selective use of their results in promotion of their conclusion.
The second part of the argument of Goldsmith et al. consists of an analysis of ozone records and a search for reductions that might have been caused by the injection of NOx. In their opinion the records show no evidence for such a change: "Analysis of the ozone records reveal no detectable changes in the total atmospheric ozone during and after the periods of nuclear weapon testing." (page 551, column 1). This wording suggests that there was either no change in ozone due to the tests, or if there was a change then it was such as to be undetected by their analysis. This is a reasonable interpretation, since it would be unrealistic to claim that even an undetectable change did not exist.
However, both the qualification that any change in ozone was undetected by their analysis and the qualifications concerning equivalence of the two types of NOx injection are missing from the abstract (the boxed bold face statement) at the beginning of the article, which states: "Although amounts of nitrogen oxides equivalent to the output from many Concordes were released into the atmosphere when nuclear testing was at its peak, the amount of ozone in the atmosphere was not affected." The word equivalent is not qualified, and the conclusion that there was no detectable change becomes one that there was no change, full stop.
The abstract easily could have been changed (within Nature's editorial requirement of less than 50 words) to include the qualifications. An alternative is, "Although nitrogen oxides equivalent in amount (if not in their effect on ozone) to the output of many Concordes probably were released into the atmosphere when nuclear testing was at its peak, our analysis reveals no detectable correlated changes in total atmospheric ozone." The reader may judge whether this qualified abstract pushes the argument of Goldsmith et al. as much as the actual one.
Out of his primary results, Johnston selects the most dramatic one, a 50% reduction in ozone, for emphasis throughout his paper. In particular, it is featured in the abstract and the conclusion, which are generally the most widely read parts of any scientific paper.
Goldsmith et al. tend to ignore, by not mentioning them in their abstract and summaries, two important qualifications to their argument: that equivalent amounts of NOx produced by SSTs and by nuclear tests may not have equivalent effects on ozone; and that although they detected no changes in ozone apparently due to nuclear test-NOx, this does not mean that no such changes occurred.
Although Johnston selectively emphasises his result giving the greatest ozone reduction, this does not necessarily mean that it is an incorrect result. Similarly, the unmentioned qualifications on the conclusion of Goldsmith et al. may not turn out upon more detailed study to be important. Once again pushing and correctness are not incompatible.
As in most scientific work, in Johnston's and in Goldsmith et al.'s work there are a host of assumptions, qualifications and judgements. Seldom can the limitations of the work be suggested fully and simply. Therefore there usually exists the possibility of pushing a scientific argument by the way these assumptions, qualifications and judgements are expressed.
Results for the variants of Johnston's standard ozone model are found in his unpublished report (1971 a). See also Johnston (1974b).
In the June 7, 1974 issue of Nature, page iii, the guide to authors states, among other things, "Articles should be accompanied by an abstract of not more than fifty words, and the abstract should list the main conclusions that are drawn."
It is interesting that the early version of Goldsmith et al.'s paper (1973a) does not contain the same abstract as the Nature version. Instead, the early version contains a long summary. The first paragraph of the summary is essentially the first three sentences of the second paragraph of the paper. The second paragraph includes one of the qualifications noted above - that no significant changes in ozone were found by Goldsmith et al.'s analysis.
There are various ways in which scientists can refer to arguments, other than their own, which are relevant to their work. In presenting evidence for an hypothesis and arguing in its favour, a scientist can attempt to take into account significant research work which supports or contradicts that hypothesis. This might take the form of an acknowledgement of work done by others or of opinions expressed, or be a closely argued consideration of an alternative viewpoint. The scientist's approach to other relevant work reflects judgements of the value of the other works, their depth of study and their pertinence to the matter at hand.
It is relatively easy for scientists to give a misleading impression of the importance of the work of others or of themselves, and indeed it is difficult not to do this. This means that it is easy for a scientific argument to be promoted by the way in which alternative arguments are referred to. The pushing of an argument in this way as a rule is not consciously planned.
Johnston in two cases puts information inconvenient to his own argument into the reference notes which are collected at the end of his paper. Let me consider these two cases.
In the body of his paper Johnston emphasises two conclusions of the SCEP report: "(i) NOx from the SST would built up to mole fraction values between 6.8 x 10-9 and 6.8 x 10-8 in the stratosphere, and (ii) these amounts of NOx may be neglected.' " (page 522, column 1). Johnston presents strong arguments that these two conclusions are incompatible. However, he does not emphasise that the figures of 6.8 x 10-9 and 6.8 x 10-8, which he refers to, are in the SCEP report itself qualified figures.
These two figures of NOx mole fractions are found using an emission ratio for SSTs of 1000 parts per million (ppm). In the SCEP report, a detailed note concerning this figure of 1000 ppm comments that it is probably too high: an estimated correction would give NOx emissions of a third to half as much, and actual measurements have found emissions to be 10% to 15% of the listed value, i.e. 100 to 150 ppm. Johnston in a long note covers these facts, and concludes it saying "at certain points I emphasize the situation based on 350 ppm of NOx." (note 7, page 522). As I have noted, he indeed emphasises the situation based on 350 ppm of NOx. He emphasises repeatedly that the values he uses are 35% of the SCEP values. And he emphasises that the SCEP mole fractions of 6.8 x 10-9 and 6.8 x 10-8 were accepted as harmless. In none of this (except in note 7, referred to above) does he mention that the SCEP estimates are qualified estimates. By relegating qualifying evidence to a note he may be considered to be pushing his argument.
Towards the beginning of his paper, Johnston refers to the work of Park and London. These workers presented results indicating that water emitted by SSTs has only a small effect on ozone. He leaves to a note the following statement: "J. Park and J. London ... also presented preliminary calculations on the effect of NO and NO2 on stratospheric O3, but I do not accept their calculations as presented." (note 5, page 522). This is the extent of his consideration of these "preliminary" calculations. His non-acceptance of their calculations may be well grounded. But by putting the reference to a conclusion opposite to his own in a note and downgrading the extent of disagreement between 'experts', he tends to push his own argument.
Johnston very kindly gave me detailed comments on the first draft of the chapters in part II. In this chapter I have not changed my original analysis of Johnston's references to alternative arguments, so it is only fair to briefly mention Johnston's comments.
Johnston says that the qualifications to SCEP's emission figures are beside the point, because the U.S. aircraft designers and their government sponsors were proceeding on the basis of the idea that stratospheric NOx mole fractions of 6.8 x 10-8 could be neglected. He says that it was not his job to speculate that the level of NOx in SST exhaust might be less than the designers thought it would be. Concerning the work of Park and London, Johnston had privately pointed out an error which invalidated their original calculations and conclusions. Johnston felt it would be unethical to use their unpublished data in a detailed presentation and unkind to headline their error.
Jumping ahead to my argument in part IV, I would say that these comments by Johnston reinforce my opinion that there is much more to scientific arguments than 'scientific' considerations. In this case politics and ethics entered in. There's nothing wrong with this. But if the argument is looked at as a purely 'scientific' one, then it becomes or appears to become a pushed argument.
Goldsmith et al. in some cases downgrade the importance of studies leading to conclusions opposite to their own by the selective use of pejorative words. For example, they sometimes refer to the arguments of others as "speculation", whereas this label is never used concerning their own work. In the introductory section of their paper they say "there has been much speculation recently that the oxides of nitrogen ...", and indicate references to work by Johnston and Crutzen. By contrast, arguing from their own results, they comment "the conclusion ... seems inescapable." (page 551, column 1). It is not obvious to me that the evidence supporting their "inescapable" conclusion is any greater than that supporting the "speculation" of Johnston and Crutzen.
Following the initial statement referring to the work of Johnston and Crutzen, Goldsmith et al. say, "It is fair to say that none of these papers contains the necessary full, quantitative consideration of the interaction of radiation, photochemistry and the atmospheric circulation." (page 545, column 1). This might seem to imply that their own study overcomes these limitations. However they qualify their conclusion by the phrase "Although the two modes of nitrogen oxide injection may not be identical from the meteorological view point, ... (page 551, column 1). So it would seem by their own admission that their "inescapable" conclusion is no more backed by a "necessary full, quantitative consideration" than are the "speculations" of Johnston and Crutzen.
The central argument of Goldsmith et al. is that thermonuclear explosions introduced large amounts of NOx into the stratosphere, and that there was no significant effect on the observed ozone distributions due to this introduced NOx. To support part of this argument they study ozone records, looking for evidence of a modification of ozone in the relevant time periods. They conclude: "It seems to us that these records do not provide evidence for such a modification." (page 550, column 1). Goldsmith et al. then discuss in the next few paragraphs the work of Johnston et al. In an independent study of the effect of NOx introduced by thermonuclear explosions on ozone and the study of ozone records, Johnston et al. conclude that "the oxides of nitrogen from nuclear bomb tests of 1952-62 constituted a measurable injection and the consequent reductions of ozone may be ascribable (perhaps only in part) to this injection experiment" (Goldsmith et al., page 550, column 1). Concerning the work of Johnston et al., Goldsmith et al. say, "It has, however, been suggested by Johnston et al. on the basis of an analysis of the records of ozone measuring stations from 1960, including those in the Soviet Union and some in the southern hemisphere, that in the northern hemisphere there was a significant decrease (-7.6% per decade) in ozone during 1960-62, followed by an increase (+5.6% per decade) in 1963-70. " (page 550, column 1). Note that according to Goldsmith et al., Johnston et al. have only "suggested" these results. Goldsmith et al. dismiss the argument of Johnston et al. in these words: "The ozone data going back to late 1957 given in Fig. 3, do not support the contention that the period 1960-62 is of any particular significance with respect to the periods of nuclear testing." (page 550, column 1). In doing this they use their own data to refute an argument based on Johnston et al.'s data, without demonstrating that their own data is superior for the task at hand. Furthermore, they suggest that while Johnston et al. claim that the ozone decrease in 1960-1962 is "significant", they (Goldsmith et al.) have shown this not to be so. Actually, as closely as one can tell from Goldsmith et al.'s Figure 4, the percentage change per decade for the period 1960-62, which is represented by the point at the middle of 1961, is -10%, a larger decrease than found by Johnston et al.! Finally, Goldsmith et al. suggest that they have demonstrated that there is no basis for Johnston et al.'s argument, when actually Johnston et al.'s argument is only that the 1960-62 ozone decrease could have resulted (perhaps only in part) from nuclear testing: even if the 1960-62 decrease is not significant, Johnston et al.'s argument is not refuted.
In my opinion, the whole tenor of Goldsmith et al.'s section about ozone records unfairly downgrades the quality and significance of Johnston et al.'s work. And since Johnston et al. come to a different conclusion than Goldsmith et al., this helps push Goldsmith et al.'s argument.
Next, consider Goldsmith et al.'s references to arguments concerning their technical assumptions. They make a number of comments in support of their technical assumptions, but do not mention arguments which cast doubt on them. One of Goldsmith et al.'s technical assumptions is that if massive amounts of NOx are injected into the stratosphere, the presence or absence of a resulting effect on stratospheric ozone will not depend sensitively on the time or place of the injection. This assumption then leads with their other results to the "equivalence" of NOx injections by Concorde and by nuclear weapons tests.
Goldsmith et al. do not make any calculations to determine the validity of their technical assumption. They do offer a few comments in justification. They note that the nitrogen oxides produced by nuclear explosives are injected at a wide range of altitudes, probably including significant amounts at the altitude range of the ozone maximum, while the nitrogen oxides injected by Concordes flying at 17 km will most likely stabilise well below the ozone maximum. Their comments imply (though do not explicitly state) that a given amount of NOx will if anything have more of an effect on ozone if injected by a representative nuclear test than if injected by Concordes.
Goldsmith et al. do not mention that there are a number of quite relevant counterarguments to this conclusion, counterarguments which were either explicit or readily apparent in previously available studies.* By ignoring these complications Goldsmith et al. suggest that their arguments are less disputable than is actually the case.
* There are a number of points that Goldsmith et al. do not consider, all of which suggest that SST-NOx deposited at low attitudes in the stratosphere may be more effective in reducing total ozone than nuclear test-NOx introduced at high altitudes. First, most estimates of the naturally occurring background of NOx give much higher mole fractions in the upper atmosphere than in the lower stratosphere. (See for example Johnston's Table 1, item 27.) Therefore stratospheric ozone may be more sensitive to a given amount of NOx added at lower altitudes than to the same amount of NOx added at higher altitudes. Second, Goldsmith et al. do not mention that ozone at high altitudes, say above 35 kin, tends to be fairly close to photochemical equilibrium, while at low altitudes, for example at 20 km, the ozone concentration is governed more by transport mechanisms. The presence of additional NOx at high altitudes establishes a new equilibrium level, but the ozone is not reduced below this. Additional NOx at low altitudes, on the other hand, may catalytically destroy ozone for a long period of time with no limit like the photochemical equilibrium. Third, if the ozone concentration at high altitudes is reduced, ozone concentrations at lower altitudes may be increased as a result. The reduction in ozone at a given high altitude means that there is less absorption of incident ultraviolet light at that altitude. Therefore more ultraviolet will penetrate to lower altitudes and be absorbed there, leading to ozone production which partially compensates in terms of the total ozone column for the reduction in ozone above. However at a low altitude of say 20 km this compensation process will be insignificant, since photochemical creation and destruction of ozone at this altitude is not important. Fourth, Goldsmith et al. do not consider the times and locations of the injections of NOx. A detailed study of the effects on ozone of NOx from nuclear tests should consider the effect of the time and location of important blasts. For example, it is possible that a blast might occur at a high latitude (near a pole) during the winter. The lack of sunlight then would make the catalytic effect of the NOx much less than if the blast occurred near the equator.
The tenor or mood of a scientific paper can help promote a particular attitude to a problem or promote a particular conclusion. The mood is created by the vocabulary, the style, the organisation of arguments, the amount of discussion of implications, etc. The mood of a scientific paper can best be judged just by reading it. There is obviously a difference in mood between Johnston's paper and Goldsmith et al.'s paper. Rather than trying to describe this difference in detail, I merely list here a few telling differences in vocabulary.
"ozone shield" (abstract)
"
burden of NOx" (page 521)
"threat to stratospheric O3 " (page 517)
"permitting the harsh radiation ... to permeate the lower atmosphere" (abstract)
"ozone layer" (page 545)
"amounts of NO" (page 549)
"interact with, and so attenuate" (page 545)
"increase the ultraviolet radiation reaching the planetary surface" (page 545)
By referring to ozone as a "shield" and talking about the "threat" to this "shield" posed by SST-NOx, Johnston reminds the reader of the possible danger to life which would result from reductions in ozone caused by human impact. Goldsmith et al. on the other hand uses words such as "layer" and "interact" for the same concepts, and thereby draw attention only to the technical aspect of the impact of SST-NOx. Such differences in vocabulary appear in relatively few places, but they are suggestive nonetheless.
A scientist may push an argument by the choice of which arguments and counterarguments to refer to, and also by the way these arguments are referred to. Johnston in a couple of cases sticks references to inconvenient or challenging material in the notes at the end of his paper. Goldsmith et al. tend to refer to the arguments of those who they dispute as "speculation". Also, they mention arguments supporting one of their technical assumptions, but not arguments casting doubt on it. Finally, the tenor or mood of each paper tends to reinforce the conclusions arrived at.